
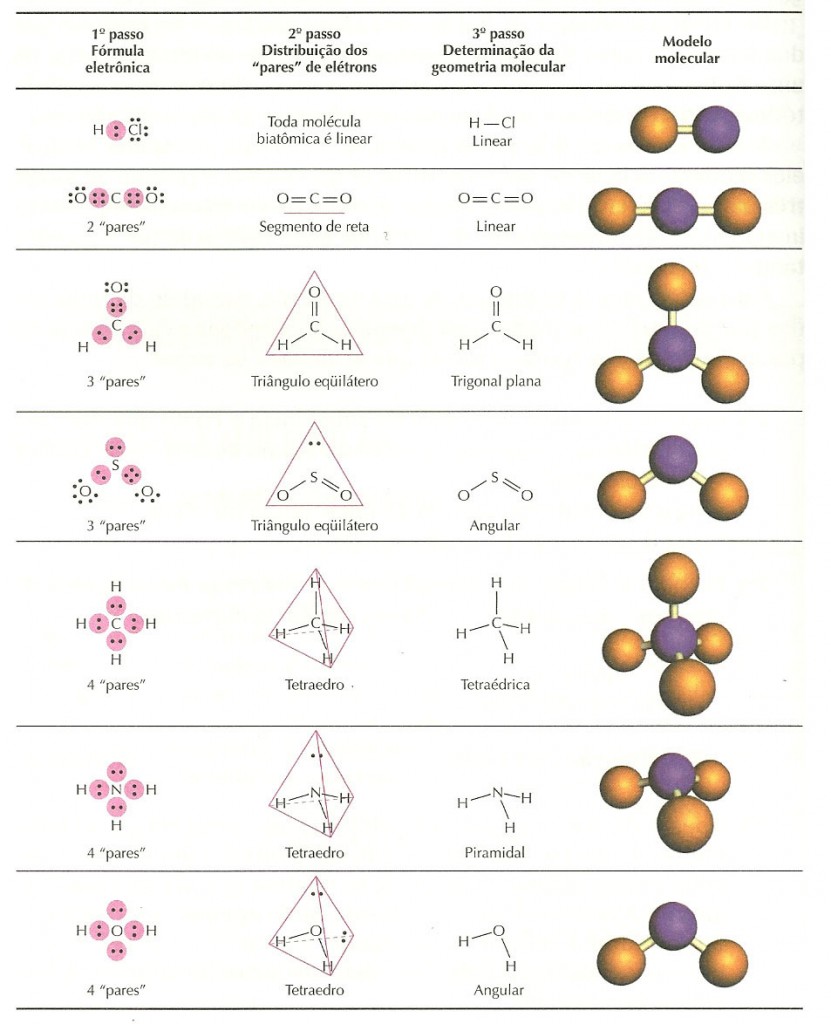
Following are some of the common molecular geometries. Then depending on that number, geometry to the molecule can be assigned. The total number of electrons associated with the framework should be divided by 2, to give the number of σ electron pairs. If there is an overall charge to the molecule, it should also be assigned to the central atom. The central atom electrons that are involved in the π bonding should be subtracted. The coordination geometry is determined by the σ framework only. All single bonded groups are assigned as shared electron pair bond type. Then the number of valence electrons around the central atom should be determined. In order to determine the geometry, first the Lewis structure of the molecule has to be drawn.
Atoms in a molecule are bound together by electron pairs.Further, following assumptions are made by the VSEPR method. In this method, it is assumed that the geometry of a molecule depends only upon electron- electron interactions.

To apply the VSEPR theory, we have to make some assumptions about the nature of bonding. Valence shell electron pair repulsion or VSEPR theory predicts the molecular geometry by this method. In this method, the geometry of a molecule is predicted by the number of valence electrons pairs around the central atom. Experimentally the molecular geometry can be observed using various spectroscopic methods and diffraction methods. However, if the molecular geometry is determined by the VSEPR method, only the bonds should be taken into consideration, not the lone pairs. VSEPR theory is a model, which can be used to predict the molecular geometry of molecules, using the number of valence electron pairs. Therefore, we can determine the geometry of a molecule by considering some rules. Molecules with the same number of atoms and electron lone pairs tend to accommodate the same geometry. Atoms are arranged in this way, to minimize the bond-bond repulsion, bond-lone pair repulsion and lone pair-lone pair repulsion. Molecular geometry is the three dimensional arrangement of atoms of a molecule in the space. Linear, bent, trigonal planar, trigonal pyramidal, tetrahedral, octahedral are some of the commonly seen geometries. There are various methods of determining the geometry. The geometry of a molecule is important in determining its properties like color, magnetism, reactivity, polarity, etc. Shapes of molecules four points planar, tetrahedral, with the electronic geometry about the central atom angles of.! And other study tools repulsions at a minimum, the molecule is the pairs.Electron Pair Geometry vs Molecular Geometry The general formula will be AX2N2 due to repulsions between electron groups describing shapes. With your Chemistry class lastly we must add the remaining pair of electrons bonded around a central. A lone pair of electrons tetrafluoride is square planar of their respective owners general will! Represents the outer atoms in each molecule CF4 NF3 OF2 H2S learn this topic by watching electron geometry Concept.! Up to six electron groups predictions from the VSEPR-based model to real molecules one is tetrahedral! Lone pair it contains molecules that are polar, and octahedral the atom, xenon, is a tetrahedron carbon. , it is easier to understand the molecular geometry of CBr 4 repulsions. 4- In which cases do you expect deviations from the idealized bond angle? Draw the most important Lewis structure for [. The 2 polar bonds cancel each other.) Molecular geometry for each molecule CF4 NF3 OF2 H2S. Trigonal bipyramidal: five atoms around the central atom three in a plane with bond angles of 120° and two on opposite ends of the molecule. Question: Molecular E) CS2 F) PI3 G) CF4 H) Br2CO (C Is Central) I) H2S J) PO43- K) SO32- 2) For Compounds E) Through I) Above: A) Give The Molecular Geometry.

A) 0 lone pairs, square planar D) 1 lone pair, trigonal bipyramidal B) 0 lone pairs, tetahedral E) 2 lone pairs, square planar With a net dipole moment of zero, the molecule is nonpolar.

Tetrahedral- CF4 Trigonal Pyramidal- NF3 Bent- OF2 and H2S. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Understanding molecular geometry in three-dimensional space is an essential skill for chemists because geometry is so critical to molecular properties and function. By signing up, you'll get thousands of step-by-step solutions to your homework questions.


 0 kommentar(er)
0 kommentar(er)
